WHO estimates that there are 3 to 5 million cases of severe illness and between 290,000 to 650,000 respiratory deaths annually. Yet current prevention tools still face major limitations: seasonal flu vaccines depend on strain prediction, offer weaker protection for people with compromised immune systems (such as older adults and cancer patients), and cannot adequately address unexpected viral variants.
In November 2025, Merck announced the acquisition of Cidara Therapeutics Inc. in an all-cash deal at $221.5 per share, valuing the transaction at $9.2 billion. The acquisition centers around Cidara's lead candidate CD388, an investigational, long-acting, and strain-agnostic antiviral agent currently advancing through Phase III clinical trials.
What is CD388?
CD388 is a first-in-class Drug–Fc Conjugate (DFC) that consists of a small molecule neuraminidase inhibitor (zanamivir) stably conjugated to a proprietary Fc fragment of a human antibody via a PEG-based linker.
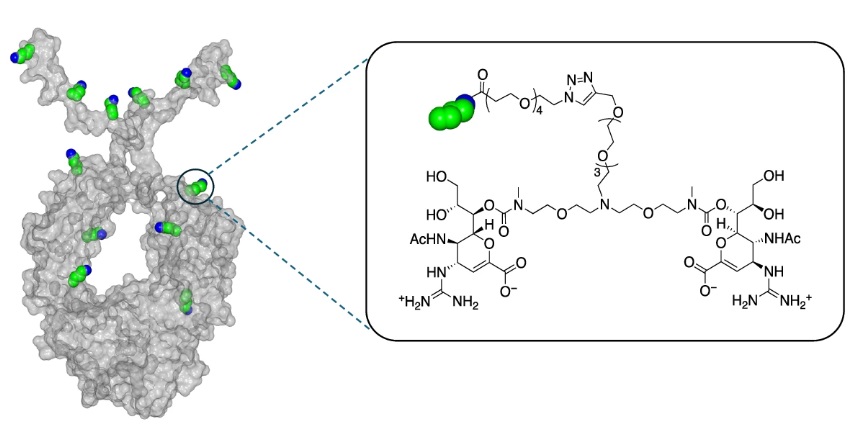
Figure 1. Structure of CD388, source: reference [2]
CD388 is not a vaccine. Unlike vaccines that stimulate an immune response to specific flu strains, CD388’s activity is not dependent on an immune response, instead, it works by directly inhibiting viral replication, preventing the virus from spreading once it enters the body.
CD388 has the potential to offer significant advantages over current flu vaccines:
- l A single dose to provide broad and durable protection against all influenza A and B strains, including highly pathogenic variants such as H5N1 for all people, including those with a compromised immune system.
- l Near-immediate protective effects.
Phase 2b NAVIGATE Trial
In June 2025, Cidara announced positive topline results from its randomized, double-blind, placebo-controlled Phase 2b NAVIGATE trial evaluating CD388 for the prevention of seasonal influenza in healthy unvaccinated adults aged 18 to 64. Single doses of 450mg, 300mg and 150mg of CD388 conferred 76%, 61% and 58% protection, respectively, from symptomatic influenza over 24 weeks compared to placebo.
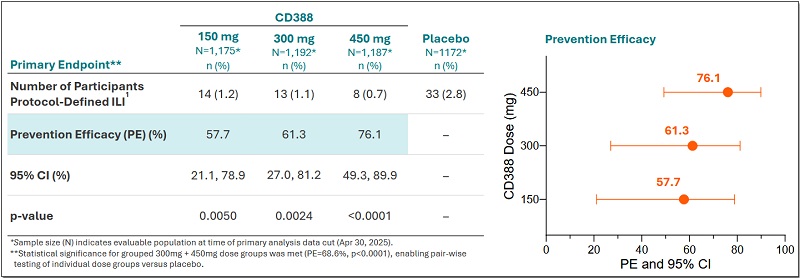
Figure 2.Phase 2b NAVIGATE trial, source: reference [4]
Based on the results, the U.S. FDA has granted CD388 Breakthrough Therapy Designation for seasonal influenza prevention. In June 2023, CD388 was granted Fast Track Designation by the FDA.
CD388 is currently being evaluated in the phase 3 ANCHOR trial (NCT07159763) in adult and adolescent patients who are at higher risk of developing complications from influenza. The trial aims to enroll 6,000 participants across high-risk populations, including adults over 65 and individuals with compromised immune systems or co-morbidities.
About Biopharma PEG
Biopharma PEG is a leading worldwide PEG linker supplier that is dedicated to manufacturing and supplying kilogram-scale manufacture of PEG derivatives in both GMP and non-GMP grades for your drug development, including monodispersed PEGs, polydispersed PEGs and multi-arm PEGs, etc.
We have a large stock of azide monodispersed PEGs to support your drug development. Visit here to find more.m
References:
[1] https://www.merck.com/news/merck-to-acquire-cidara-therapeutics-inc-diversifying-its-portfolio-to-include-late-phase-antiviral-agent/
[2] Döhrmann, S., Levin, J., Cole, J. N., Borchardt, A., Amundson, K., Almaguer, A., Abelovski, E., Grewal, R., Zuill, D., Dedeic, N., Hough, G., Fortier, J., Donatelli, J., Lam, T., Chen, Z., Jiang, W., Haussener, T., Noncovich, A., Balkovec, J. M., . . . Tari, L. W. (2025). Drug–Fc conjugate CD388 targets influenza virus neuraminidase and is broadly protective in mice. Nature Microbiology, 10(4), 912-926. https://doi.org/10.1038/s41564-025-01955-3
[3] https://www.cidara.com/news/cidara-therapeutics-announces-positive-topline-results-from-its-phase-2b-navigate-trial-evaluating-cd388-a-non-vaccine-preventative-of-seasonal-influenza/
[4] https://seekingalpha.com/article/4831516-cidara-therapeutics-a-potential-blockbuster-in-flu-prevention

